A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables
- 15 October 2023
- Grondin Eric
- (0)
- Lacto-fermentation, Probiotics, Scientific paper
This blog article was written by the authors below and is shared in accordance with the Creative Commons CC BY license. No modifications have been made to its English version. If you choose the French language, an automatic translation by Google Translate is provided within the article.
Copyright : © 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Abstract
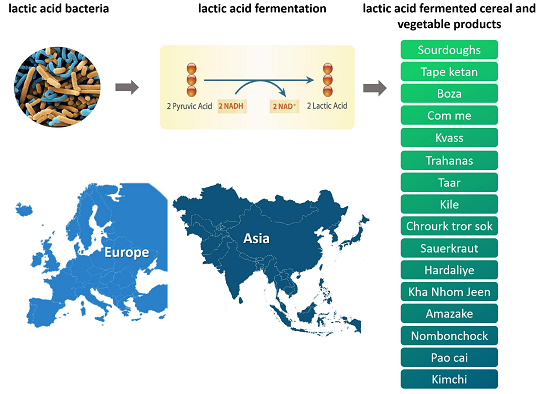
Lactic acid fermentation is one of the oldest methods used worldwide to preserve cereals and vegetables. Europe and Asia have long and huge traditions in the manufacturing of lactic acid bacteria (LAB)-fermented foods. They have different cultures, religions and ethnicities with the available resources that strongly influence their food habits. Many differences and similarities exist with respect to raw substrates, products and microbes involved in the manufacture of fermented products. Many of them are produced on industrial scale with starter cultures, while others rely on spontaneous fermentation, produced homemade or in traditional events. In Europe, common LAB-fermented products made from cereals include traditional breads, leavened sweet doughs, and low and non-alcoholic cereal-based beverages, whereas among vegetable ones prevail sauerkraut, cucumber pickles and olives. In Asia, the prevailing LAB-fermented cereals include acid-leavened steamed breads or pancakes from rice and wheat, whereas LAB-fermented vegetables are more multifarious, such as kimchi, sinki, khalpi, dakguadong, jiang-gua, soidon and sauerkraut. Here, an overview of the main Euro-Asiatic LAB-fermented cereals and vegetables was proposed, underlining the relevance of fermentation as a tool for improving cereals and vegetables, and highlighting some differences and similarities among the Euro-Asiatic products. The study culminated in “omics”-based and future-oriented studies of the fermented products.
Keywords: lactic acid bacteria; fermented foods; cereals; vegetables; Europe; Asia; beverages; starter cultures; rice; wheat
1. Introduction
2. Fermentation as a Tool for Improving Cereals and Vegetables
3. Lactic Acid Bacteria (LAB) Fermentation
4. European LAB-Fermented Cereals and Vegetables
4.1. Fermented Cereals
Table 1. Selected lactic acid bacteria fermented cereals and vegetables in Euro-Asia.
A1. Fermented European Cereal Products
A2. Fermented European Vegetables Products
B1. Fermented Asian Cereal Products
B2. Fermented Asian Vegetable Products
* In the Table 1, we keep the names of the old classification of the genus Lactobacillus to avoid any confusion in the reader. However, the links for the appropriate conversions are as follows: http://lactobacillus.ualberta.ca; http://lactobacillus.uantwerpen.be; http://lactotax.embl.de/wuyts/lactotax/).
4.2. Fermented Vegetables
5. LAB-Fermented Cereals and Vegetables of Asian Origin
5.1. Fermented Cereals
5.2. Fermented Vegetables
6. Prospective Studies and Considerations
7. Conclusions
Conceptualization, writing—original draft preparation, writing—review and editing, supervision, A.R. and T.J.A. These authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
This study was supported by the Project PRIN 2017 “The Neapolitan pizza: processing, distribution, innovation and environmental aspect” (2017SFTX3Y), funded by the Italian Ministry of Instruction, University and Research (MIUR) and by CNR project NUTR-AGE (FOE-2019, DSB.AD004.271). The authors are also grateful to the Smart Agriculture Research and Application Team, Faculty of Applied Sciences, Ton Duc Thang University, Vietnam.
The authors declare no conflict of interest.
- Díaz, L.D.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Ashaolu, T.J. A review on selection of fermentative microorganisms for functional foods and beverages: The production and future perspectives. Int. J. Food Sci. Technol. 2019, 54, 2511–2519. [Google Scholar] [CrossRef]
- Simatende, P.; Gadaga, T.H.; Nkambule, S.J.; Siwela, M. Methods of preparation of Swazi traditional fermented foods. J. Ethn. Foods 2015, 2, 119–125. [Google Scholar] [CrossRef][Green Version]
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- Neves, A.; Becker, W.; Dominguez-Torreiro, M. Explained, the Economic Ties between Europe and Asia. World Economic Forum. 2019. Available online: https://www.weforum.org/agenda/2019/05/ways-asia-and-europe-together-connected/#:~:text=Asia%2DEurope%20trade,other%20regions%20in%20the%20world (accessed on 19 June 2020).
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Thai Fermented Foods as a Versatile Source of Bioactive Microorganisms—A Comprehensive Review. Sci. Pharm. 2018, 86, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammes, W.P.; Gänzle, M.G. Sourdough breads and related products. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Blackie Academic and Professional: London, UK, 1998; pp. 199–216. [Google Scholar]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steinkraus, K. Fermentations in World Food Processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Safety and quality of bacterially fermented functional foods and beverages: A mini review. Food Qual. Saf. 2020. [Google Scholar] [CrossRef]
- Ray, R.C.; Sivakumar, P.S. Traditional and novel fermented foods and beverages from tropical root and tuber crops. Int. J. Food Sci. Technol. 2009, 44, 1073–1087. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.; Pandiella, S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Messia, M.C.; Reale, A.; Maiuro, L.; Candigliota, T.; Sorrentino, E.; Marconi, E. Effects of pre-fermented wheat bran on dough and bread characteristics. J. Cereal Sci. 2016, 69, 138–144. [Google Scholar] [CrossRef]
- Reale, A.; Mannina, L.; Tremonte, P.; Sobolev, A.P.; Succi, M.; Sorrentino, E.; Coppola, R. Phytate Degradation by Lactic Acid Bacteria and Yeasts during the Wholemeal Dough Fermentation: a31P NMR Study. J. Agric. Food Chem. 2004, 52, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Konietzny, U.; D’Auria, S.; Sorrentino, E.; Greiner, R. The Importance of Lactic Acid Bacteria for Phytate Degradation during Cereal Dough Fermentation. J. Agric. Food Chem. 2007, 55, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2017, 59, 506–527. [Google Scholar] [CrossRef]
- Di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavoring Production in Kamut®, Quinoa and Wheat Doughs Fermented by Lactobacillus paracasei, Lactobacillus plantarum, and Lactobacillus brevis: A SPME-GC/MS Study. Front. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef][Green Version]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef][Green Version]
- Terefe, N.S. Food Fermentation. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Saibandith, B.; Yupanqui, C.T.; Wichienchot, S. Human colonic microbiota modulation and branched chain fatty acids production affected by soy protein hydrolysate. Int. J. Food Sci. Technol. 2018, 54, 141–148. [Google Scholar] [CrossRef][Green Version]
- Nuraida, L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci. Hum. Wellness 2015, 4, 47–55. [Google Scholar] [CrossRef][Green Version]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A. Traditional low-alcoholic and non-alcoholic fermented beverages consumedin European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Microbiological and Fermentative Properties of Baker’s Yeast Starter Used in Breadmaking. J. Food Sci. 2013, 78, M1224–M1231. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Di Renzo, T.; Boscaino, F.; Nazzaro, F.; Fratianni, F.; Aponte, M. Lactic Acid Bacteria Biota and Aroma Profile of Italian Traditional Sourdoughs from the Irpinian Area in Italy. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gobbetti, M.; Minervini, F.; Pontonio, E.; Di Cagno, R.; De Angelis, M. Drivers for the establishment and composition of the sourdough lactic acid bacteria biota. Int. J. Food Microbiol. 2016, 239, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Di Cagno, R.; Lattanzi, A.; De Angelis, M.; Antonielli, L.; Cardinali, G.; Cappelle, S.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical italian breads: Interactions between ingredients and microbial species diversity. Appl. Environ. Microbiol. 2011, 78, 1251–1264. [Google Scholar] [CrossRef][Green Version]
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Identification of lactobacilli isolated in traditional ripe wheat sourdoughs by using molecular methods. World J. Microbiol. Biotechnol. 2011, 27, 237–244. [Google Scholar] [CrossRef]
- Zotta, T.; Piraino, P.; Parente, E.; Salzano, G.; Ricciardi, A. Characterization of lactic acid bacteria isolated from sourdoughs for Cornetto, a traditional bread produced in Basilicata (Southern Italy). World J. Microbiol. Biotechnol. 2008, 24, 1785–1795. [Google Scholar] [CrossRef]
- Robert, H.; Gabriel, V.; Fontagné-Faucher, C. Biodiversity of lactic acid bacteria in French wheat sourdough as determined by molecular characterization using species-specific PCR. Int. J. Food Microbiol. 2009, 135, 53–59. [Google Scholar] [CrossRef]
- Reale, A.; Tremonte, P.; Succi, M.; Sorrentino, E.; Coppola, R. Exploration of lactic acid bacteria ecosystem of sourdoughs from the Molise region. Ann. Microbiol. 2005, 55, 17–22. [Google Scholar]
- De Vuyst, L.; Schrijvers, V.; Paramithiotis, S.; Hoste, B.; Vancanneyt, M.; Swings, J.; Kalantzopoulos, G.; Tsakalidou, E.; Messens, W. The Biodiversity of Lactic Acid Bacteria in Greek Traditional Wheat Sourdoughs Is Reflected in Both Composition and Metabolite Formation. Appl. Environ. Microbiol. 2002, 68, 6059–6069. [Google Scholar] [CrossRef][Green Version]
- Pepe, O. Effect of proteolytic starter cultures as leavening agents of pizza dough. Int. J. Food Microbiol. 2003, 84, 319–326. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microbiol. 2012, 31, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. On the microbiological profile of traditional portuguese sourdough. J. Food Prot. 1999, 62, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. Microbial Ecology Dynamics in Portuguese Broa Sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Catzeddu, P. Flour and Breads and Their Fortification in Health and Disease Prevention; Sourdough breads; Academic Press: Cambridge, MA, USA, 2011; pp. 37–46. [Google Scholar]
- Viiard, E.; Bessmeltseva, M.; Simm, J.; Talve, T.; Aaspõllu, A.; Paalme, T.; Sarand, I. Diversity and stability of lactic acid bacteria in rye sourdoughs of four bakeries with different propagation parameters. PLoS ONE 2016, 11, e0148325. [Google Scholar] [CrossRef][Green Version]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2019, 8, 64. [Google Scholar] [CrossRef][Green Version]
- Coda, R.; Nionelli, L.; Rizzello, C.G.; De Angelis, M.; Tossut, P.; Gobbetti, M. Spelt and emmer flours: Characterization of the lactic acid bacteria microbiota and selection of mixed starters for bread making. J. Appl. Microbiol. 2010, 108, 925–935. [Google Scholar] [CrossRef]
- Moroni, A.V.; Arendt, E.K.; Bello, F.D. Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiol. 2011, 28, 497–502. [Google Scholar] [CrossRef]
- Korcari, D.; Ricci, G.; Quattrini, M.; Fortina, M.G. Microbial consortia involved in fermented spelt sourdoughs: Dynamics and characterization of yeasts and lactic acid bacteria. Lett. Appl. Microbiol. 2019, 70, 48–54. [Google Scholar] [CrossRef]
- Harth, H.; Van Kerrebroeck, S.; De Vuyst, L. Impact of process conditions on the microbial community dynamics and metabolite production kinetics of teff sourdough fermentations under bakery and laboratory conditions. Food Sci. Nutr. 2018, 6, 1438–1455. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Gambuś, H.; Mickowska, B.; Bartoń, H.; Augustyn, G.; Zięć, G.; Litwinek, D.; Szary-Sworst, K.; Berski, W. Health benefits of kvass manufactured from rye wholemeal bread. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 34–39. [Google Scholar] [CrossRef]
- Georgala, A. The Nutritional Value of Two Fermented Milk/Cereal Foods Named ‘Greek Trahanas’ and ‘Turkish Tarhana’: A review. J. Nutr. Disord. Ther. 2012, S11, 002. [Google Scholar] [CrossRef][Green Version]
- Lazos, E.S.; Aggelousis, G.; Bratakos, M. The fermentation of trahanas: A milk-wheat flour combination. Plant Foods Hum. Nutr. 1993, 44, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Garofalo, C.; Aquilanti, L.; Milanović, V.; Clementi, F. Unpasteurised commercial boza as a source of microbial diversity. Int. J. Food Microbiol. 2015, 194, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Altay, F.; Karbancıoğlu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gotcheva, V.; Pandiella, S.; Angelov, A.; Roshkova, Z.G.; Webb, C. Microflora identification of the Bulgarian cereal-based fermented beverage boza. Process. Biochem. 2000, 36, 127–130. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Fermented Foods, Part II.; Traditional cereal beverage boza fermentation technology, microbial content and healthy effects; CRC Press: Boca Raton, FL, USA, 2017; pp. 284–305. [Google Scholar]
- Botes, A.; Todorov, S.D.; Von Mollendorff, J.W.; Botha, A.; Dicks, L.M.T. Identification of lactic acid bacteria and yeast from boza. Process. Biochem. 2007, 42, 267–270. [Google Scholar] [CrossRef]
- Zorba, M.; Hancioglu, O.; Genç, M.; Karapinar, M.; Ova, G. The use of starter cultures in the fermentation of boza, a traditional Turkish beverage. Process. Biochem. 2003, 38, 1405–1411. [Google Scholar] [CrossRef]
- Soukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A.; et al. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef]
- Moora, A. Eesti Talurahva Vanem Toit; Valgus: Tallinn, Estonia, 2007. [Google Scholar]
- Grosu-tudor, S.; Stefan, I.; Stancu, M.; Cornea, C.; De vuyst, L.; Zamfir, M. Microbial and nutritional characteristics of fermented wheat bran in traditional Romanian borş production. Romanian Biotechnol. Lett. 2018, 24, 440–447. [Google Scholar] [CrossRef]
- Basinskiene, L.; Juodeikiene, G.; Vidmantiene, D.; Tenkanen, M.; Makaravicius, T.; Bartkiene, E. Non-Alcoholic beverages from fermented cereals with increased oligosaccharide content. Food Technol. Biotechnol. 2016, 54, 36–44. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef][Green Version]
- Dlusskaya, E.; Jänsch, A.; Schwab, C.; Gänzle, M. Microbial and chemical analysis of a kvass fermentation. Eur. Food Res. Technol. 2007, 227, 261–266. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Bordons, A.; Jiménez-Díaz, R. Editorial: New Trends in Table Olive Fermentation. Front. Microbiol. 2019, 10, 1880. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, S.C.; Panagou, E.Z.; Nychas, G.-J. Table Olive Fermentation Using Starter Cultures with Multifunctional Potential. Microorganisms. 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heperkan, D. Microbiota of table olive fermentations and criteria of selection for their use as starters. Front. Microbiol. 2013, 4, 143. [Google Scholar] [CrossRef][Green Version]
- Çoşkun, F. A Traditional Turkish Fermented Non-Alcoholic Grape-Based Beverage, “Hardaliye. ” Beverages 2017, 3, 2. [Google Scholar] [CrossRef][Green Version]
- Arici, M.; Coskun, F. Hardaliye: Fermented grape juice as a traditional Turkish beverage. Food Microbiol. 2001, 18, 417–421. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Uslu, N.; Figueredo, G.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Alsawmahi, O.N.; Ozcan, M.M.; Isam, A.; Ahmed, A. The effect of fermentation process on bioactive properties, essential oil composition and phenolic constituents of raw fresh and fermented sea fennel (Crithmum maritimum L.) leaves. Indian J. Trad. Knowl. 2019, 18, 800–804. [Google Scholar]
- Maifreni, M.; Marino, M.; Conte, L. Lactic acid fermentation of Brassica rapa: Chemical and microbial evaluation of a typical Italian product (brovada). Eur. Food Res. Technol. 2004, 218, 469–473. [Google Scholar] [CrossRef]
- Zabat, M.; Sano, W.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beganović, J.; Kos, B.; Pavunc, A.L.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, H.; Surma, M.; Zielińska, D. Chapter 21—The naturally fermented sour pickled cucumbers. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 503–516. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Phromraksa, P.; Nagano, H.; Boonmars, T.; Kamboonruang, C. Identification of Proteolytic Bacteria from Thai Traditional Fermented Foods and Their Allergenic Reducing Potentials. J. Food Sci. 2008, 73, M189–M195. [Google Scholar] [CrossRef]
- Rhee, S.J.; Lee, J.-E.; Lee, C.-H. Importance of lactic acid bacteria in Asian fermented foods. Microbial Cell Factories 2011, 10, S5. [Google Scholar] [CrossRef][Green Version]
- Pisitkul, C.; Rengpipat, S. Isolation and characterization of lactic acid bacteria starter for preparation of fermented Khanom-jeen. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Chiang Rai, Thaland, 26–29 November 2014; pp. 353–359. [Google Scholar]
- Peng, C.; Borges, S.; Magalhães, R.; Carvalheira, A.; Ferreira, V.; Casquete, R.; Teixeira, P. Characterization of anti-listerial bacteriocin produced by lactic acid bacteria isolated from traditional fermented foods from Cambodia. Int. Food Res. 2017, 24, 386–393. [Google Scholar]
- Luangsakul, N.; Keeratipibul, S.; Jindamorakot, S.; Tanasupawat, S. Lactic acid bacteria and yeasts isolated from the starter doughs for Chinese steamed buns in Thailand. LWT Food Sci. Technol. 2009, 42, 1404–1412. [Google Scholar] [CrossRef]
- La Anh, N. Health-promoting microbes in traditional Vietnamese fermented foods: A review. Food Sci. Hum. Wellness 2015, 4, 147–161. [Google Scholar] [CrossRef][Green Version]
- Çakır, E.; Arıcı, M.; Durak, M.Z.; Karasu, S. The molecular and technological characterization of lactic acid bacteria in einkorn sourdough: Effect on bread quality. J. Food Meas. Charact. 2020, 14, 1646–1655. [Google Scholar] [CrossRef]
- Kivanç, M.; Funda, E.G. A functional food: A traditional Tarhana fermentation. Food Sci. Technol. 2017, 37, 269–274. [Google Scholar] [CrossRef][Green Version]
- Hasanah, U.; Ratihwulan, H.; Nuraida, L. Sensory profiles and lactic acid bacteria density of Tape Ketan and Tape Singkong in Bogor. agriTECH 2019, 38, 265–272. [Google Scholar] [CrossRef]
- Yonzan, H.; Tamang, J.P. Microbiology and Nutritional Value ofSelroti, an Ethnic Fermented Cereal Food of the Himalayas. Food Biotechnol. 2010, 24, 227–247. [Google Scholar] [CrossRef]
- Yongsmith, B.; Malaphan, W. Traditional Foods; Traditional Fermented Foods in Thailand; Springer: Boston, MA, USA, 2016; pp. 31–59. [Google Scholar]
- Nguyen, D.T.L.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; Aerts, M.; Thanh, L.B.; Vandamme, P. A description of the lactic acid bacteria microbiota associated with the production of traditional fermented vegetables in Vietnam. Int. J. Food Microbiol. 2013, 163, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.T.L.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; Aerts, M.; Le Thanh, B.; Vandamme, P. Validation of MALDI-TOF MS for rapid classification and identification of lactic acid bacteria, with a focus on isolates from traditional fermented foods in Northern Vietnam. Lett. Appl. Microbiol. 2012, 55, 265–273. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kusumoto, K.I.; Oguma, T.; Nagai, T.; Furukawa, S.; Suzuki, C.; Satomi, M.; Magariyama, Y.; Takamine, K.; Tamaki, H. Ethnic Fermented Foods and Alcoholic Beverages of Asia; Ethnic Fermented Foods and Alcoholic Beverages of Japan; Springer: New Delhi, India, 2016; pp. 193–236. [Google Scholar]
- Tamang, J.P.; Sarkar, P.K. Sinki: A traditional lactic acid fermented radish tap root product. J. Gen. Appl. Microbiol. 1993, 39, 395–408. [Google Scholar] [CrossRef][Green Version]
- Franco, W.; Johanningsmeier, S.D.; Lu, J.; Demo, J.; Wilson, E.; Moeller, L. Cucumber Fermentation; Book Chapter; Chapter 7; CRC Press: Boca Raton, FL, USA, 2016; pp. 107–155. [Google Scholar]
- Mäki, M. Lactic Acid Bacteria in Vegetable Fermentations. Food Sci. Technol. N. Y. Marcel Dekker 2004, 139, 419–430. [Google Scholar] [CrossRef]
- Harris, L.J. Microbiology of Fermented Foods; The microbiology of vegetable fermentations; Springer: Boston, MA, USA, 1998; pp. 45–72. [Google Scholar]
- Pino, A.; De Angelis, M.; Todaro, A.; Van Hoorde, K.; Randazzo, C.L.; Caggia, C. Fermentation of Nocellara Etnea Table Olives by Functional Starter Cultures at Different Low Salt Concentrations. Front. Microbiol. 2018, 9, 1125. [Google Scholar] [CrossRef][Green Version]
- Panagou, E.Z.; Tassou, C.C.; Katsaboxakis, C.Z. Induced lactic acid fermentation of untreated green olives of the Conservolea cultivar byLactobacillus pentosus. J. Sci. Food Agric. 2003, 83, 667–674. [Google Scholar] [CrossRef]
- Aponte, M.; Ventorino, V.; Blaiotta, G.; Volpe, G.; Farina, V.; Avellone, G.; Lanza, C.M.; Moschetti, G. Study of green Sicilian table olive fermentations through microbiological, chemical and sensory analyses. Food Microbiol. 2010, 27, 162–170. [Google Scholar] [CrossRef]
- Garrido-Fernández, A. Effect of processing conditions on lactic acid bacteria growth in table olive fermentation. Actes Colloq. Lact. 1997, 97, 277–316. [Google Scholar]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodriguez-Gomez, F.; Jimenez-Diaz, R.; García-García, P.; Querol, A.; Garrido-Fernandez, A. Yeasts in table olive processing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.; Blaiotta, G.; La Croce, F.; Mazzaglia, A.; Farina, V.; Settanni, L.; Moschetti, G. Use of selected autochthonous lactic acid bacteria for Spanish-style table olive fermentation. Food Microbiol. 2012, 30, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Papadelli, M.; Zoumpopoulou, G.; Georgalaki, M.; Anastasiou, R.; Manolopoulou, E.; Lytra, I.; Papadimitriou, K.; Tsakalidou, E. Evaluation of Two Lactic Acid Bacteria Starter Cultures for the Fermentation of Natural Black Table Olives (Olea europaea L cv Kalamon). Pol. J. Microbiol. 2015, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Cabello, A.; Rodríguez-Gómez, F.; Morales, M.L.; Garrido-Fernández, A.; Jiménez-Díaz, R.; Arroyo-López, F.N. Lactic acid bacteria and yeast inocula modulate the volatile profile of spanish-style green table olive fermentations. Foods 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Touret, T.; Oliveira, M.; Semedo-Lemsaddek, T. Putative probiotic lactic acid bacteria isolated from sauerkraut fermentations. PLoS ONE 2018, 13, e0203501. [Google Scholar] [CrossRef][Green Version]
- Di Cagno, R.; Surico, R.F.; Siragusa, S.; De Angelis, M.; Paradiso, A.; Minervini, F.; De Gara, L.; Gobbetti, M. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows. Int. J. Food Microbiol. 2008, 127, 220–228. [Google Scholar] [CrossRef]
- Pulido, R.P.; Ben Omar, N.; Abriouel, H.; López, R.L.; Cañamero, M.M.M.; Guyot, J.-P.; Gálvez, A. Characterization of lactobacilli isolated from caper berry fermentations. J. Appl. Microbiol. 2007, 102, 583–590. [Google Scholar] [CrossRef]
- Pulido, R.; Benomar, N.; Cañamero, M.; Abriouel, H.; Gálvez, A.; Hui, Y.; Evranuz, E. Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; Fermentation of Caper Products; CRC Press: Boca Raton, FL, USA, 2012; pp. 201–208. [Google Scholar]
- Malinowska-Pańczyk, E. Fermented vegetables products. Food Flavors 2012. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef][Green Version]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Oupathumpanont, O.; Suwonsichon, T. Change of sensory characteristic of fermented rice flour and fermented rice noodle (Kanom-jeen) during fermentation by Lactobacillus plantarum A1. J. Appl. Sci. Res. 2013, 9, 6063–6071. [Google Scholar]
- Ly, D.; Mayrhofer, S.; Domig, K. Significance of traditional fermented foods in the lower Mekong subregion: A focus on lactic acid bacteria. Food Biosci. 2018, 26, 113–125. [Google Scholar] [CrossRef]
- Keatkrai, J.; Jirapakkul, W. Volatile profile of khanom jeen, Thai fermented rice noodles, and the changes during the fermentation process. Sci. Asia 2010, 36, 46–51. [Google Scholar] [CrossRef]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy based probiotics: A healthy treat for intestine. Crit. Rev. Food Sci. Nutr. 2015, 56, 1856–1867. [Google Scholar] [CrossRef]
- Hamajima, H.; Tanaka, M.; Miyagawa, M.; Sakamoto, M.; Nakamura, T.; Yanagita, T.; Nishimukai, M.; Mitsutake, S.; Nakayama, J.; Nagao, K.; et al. Koji glycosylceramide commonly contained in Japanese traditional fermented foods alters cholesterol metabolism in obese mice. Biosci. Biotechnol. Biochem. 2018, 83, 1514–1522. [Google Scholar] [CrossRef]
- Saito, Y.; Wanezaki, K.W.; Kawato, A. Antihypertensive Effects of Peptide in Sake and Its By-products on Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 1994, 58, 812–816. [Google Scholar] [CrossRef][Green Version]
- Jeon, H.J.; Noda, M.; Maruyama, M.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Identification and Kinetic Study of Tyrosinase Inhibitors Found in Sake Lees. J. Agric. Food Chem. 2006, 54, 9827–9833. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nakashima, Y.; Yoshikawa, J.; Wada, N.; Matsugo, S. Radical scavenging activity of the japanese traditional food, Amazake. Food Sci. Technol. Res. 2011, 17, 209–218. [Google Scholar] [CrossRef][Green Version]
- Maruki-Uchida, H.; Sai, M.; Yano, S.; Morita, M.; Maeda, K. Amazake made from sake cake and rice koji suppresses sebum content in differentiated hamster sebocytes and improves skin properties in humans. Biosci. Biotechnol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Oguro, Y.; Nishiwaki, T.; Shinada, R.; Kobayashi, K.; Kurahashi, A. Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. J. Biosci. Bioeng. 2017, 124, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, N.; Sert, D.; Demir, M.K.; Elgün, A. Effect of whey concentrate addition on the chemical, nutritional and sensory properties of tarhana (a Turkish Fermented Cereal-based Food). Food Sci. Technol. Res. 2009, 15, 51–58. [Google Scholar] [CrossRef]
- Özel, S.; Sabanoğlu, S.; Çon, A.H.; Simsek, O. Diversity and stability of yeast species during the fermentation of tarhana. Food Biotechnol. 2015, 29, 117–129. [Google Scholar] [CrossRef]
- Bayrakçı, H.A.; Bilgiçli, N. Influence of resistant starches on chemical and functional properties of tarhana. J. Food Sci. Technol. 2014, 52, 5335–5340. [Google Scholar] [CrossRef][Green Version]
- Arici, M.; Dağlıoğlu, O. Boza: A lactic acid fermented cereal beverage as a traditional Turkish food. Food Rev. Int. 2002, 18, 39–48. [Google Scholar] [CrossRef]
- Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Ozcan, T. Determination of boza’s organic acid composition as it is affected by raw material and fermentation. Int. J. Food Prop. 2010, 13, 648–656. [Google Scholar] [CrossRef]
- Sujaya, I.N.; Nocianitri, K.A.; Asano, K. Diversity of bacterial flora of Indonesian ragi tape and their dynamics during the tape fermentation as determined by PCR-DGGE. Int. Food Res. J. 2010, 17, 239–245. [Google Scholar]
- Ganzle, M.G.; Salovaara, H. Lactic acid bacteria in cereal-based products. In Lactic Acid Bacteria: Microbiological and Functional Aspects; Vinderola, G., Ouwehand, A., Salminen, S., von Wriu, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 199–208. [Google Scholar]
- Ai-Lati, A.; Liu, S.; Li, X.; Qian, B.; Shan, Y.; Zhou, Z.; Peng, L.; Ji, Z.; Mao, J.; Zou, H.; et al. Effect of Chinese rice wine sludge on the production of Chinese steamed buns. J. Food Process. Preserv. 2018, 42, e13572. [Google Scholar] [CrossRef]
- Puspito, H.; Fleet, G. Microbiology of sayur asin fermentation. Appl. Microbiol. Biotechnol. 1985, 22, 442–445. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Komagata, K. Lactic acid bacteria in fermented foods in Thailand. World J. Microbiol. Biotechnol. 1995, 11, 253–256. [Google Scholar] [CrossRef]
- Viander, B.; Mäki, M.; Palva, A. Impact of low salt concentration, salt quality on natural large-scale sauerkraut fermentation. Food Microbiol. 2003, 20, 391–395. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yanagida, F.; Hsu, J.-S. Isolation and characterization of lactic acid bacteria from suan-tsai (fermented mustard), a traditional fermented food in Taiwan. J. Appl. Microbiol. 2006, 101, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.M.; Xue, W.T.; Tan, S.S.; Zhang, H.; Chang, X.H. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control. 2008, 19, 50–55. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, Y.S.; Yanagida, F. Isolation and characterization of lactic acid bacteria from yan-jiang (fermented ginger), a traditional fermented food in Taiwan. J. Sci. Food Agric. 2011, 91, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Wu, H.C.; Lo, H.Y.; Lin, W.C.; Hsu, W.H.; Lin, C.W.; Lin, P.Y.; Yanagida, F. Isolation and characterization of lactic acid bacteria from jiang-gua (fermented cucumbers), a traditional fermented food in Taiwan. J. Sci. Food Agric. 2012, 92, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Wu, H.C.; Wang, C.M.; Lin, C.C.; Chen, Y.T.; Jhong, Y.J.; Yanagida, F. Isolation and characterization of lactic acid bacteria from pobuzihi (fermented cummingcordia), a traditional fermented food in Taiwan. Folia Microbiol. 2013, 58, 103–109. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Tamang, J.; Thapa, N.; Tamang, B.; Rai, A.; Chettri, R. Health Benefits of Fermented Foods and Beverages; Microorganisms in fermented foods and beverages; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–110. [Google Scholar]
- Medina, E.; De Castro, A.; Romero, C.; Ramírez, E.M.; Brenes, M. Safety of fermented fruits and vegetables. Regul. Saf. Tradit. Ethn. Foods 2016, 355–367. [Google Scholar] [CrossRef]
- Shin, G.H.; Kang, B.C.; Jang, D.J. Metabolic Pathways Associated with Kimchi, a Traditional Korean Food, Based onIn SilicoModeling of Published Data. Genom. Inform. 2016, 14, 222–229. [Google Scholar] [CrossRef][Green Version]
- Park, K.Y.; Kim, H.Y.; Jeong, J.K. Chapter 20—Kimchi and its health Benefits A2—Frias, Juana. In Fermented Foods in Health and Disease Prevention; Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 477–502. [Google Scholar]
- Meidong, R.; Doolgindachbaporn, S.; Jamjan, W.; Sakai, K.; Tashiro, Y.; Okugawa, Y.; Tongpim, S. A novel probiotic Bacillus siamensis B44v isolated from Thai pickled vegetables (Phak-dong) for potential use as a feed supplement in aquaculture. J. Gen. Appl. Microbiol. 2017, 63, 246–253. [Google Scholar] [CrossRef][Green Version]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašićd, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef]
- Marui, J.; Boulom, S.; Panthavee, W.; Momma, M.; Kusumoto, K.-I.; Nakahara, K.; Saito, M. Culture-independent bacterial community analysis of the salty-fermented fish paste products of Thailand and Laos. Biosci. Microbiota Food Health 2015, 34, 45–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, D.T.L.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; De Bruyne, K.; Le, B.T.; Vandamme, P. A culture-dependent and -independent approach for the identification of lactic acid bacteria associated with the production of nem chua, a Vietnamese fermented meat product. Food Res. Int. 2013, 50, 232–240. [Google Scholar] [CrossRef]
- Wanangkarn, A.; Liu, D.; Swetwiwathana, A.; Jindaprasert, A.; Phraephaisarn, C.; Chumnqoen, W.; Tan, F.J. Lactic acid bacterial population dynamics during fermentation and storage of Thai fermented sausage according to restriction fragment length polymorphism analysis. Int. J. Food Microbiol. 2014, 186, 61–67. [Google Scholar] [CrossRef]
- Moe, N.K.T.; Thwe, S.M.; Shirai, T.; Terahara, T.; Imada, C.; Kobayashi, T. Characterization of lactic acid bacteria distributed in small fish fermented with boiled rice in Myanmar. Fish. Sci. 2015, 81, 373–381. [Google Scholar] [CrossRef]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Roytrakul, S.; Perez, R.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Two putatively novel bacteriocins active against Gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control. 2015, 55, 176–184. [Google Scholar] [CrossRef]
- Jagadeesan, B.; Gerner-Smidt, P.; Allard, M.W.; Leuillet, S.; Winkler, A.; Xiao, Y.; Chaffron, S.; Van Der Vossen, J.; Tang, S.; Katase, M.; et al. The use of next generation sequencing for improving food safety: Translation into practice. Food Microbiol. 2019, 79, 96–115. [Google Scholar] [CrossRef]



Leave a Comment